Oxbryta Can Ease or Fully Heal Leg Ulcers, Trial Analysis Shows
IN NEWS.
Oxbryta (voxelotor) can help ease, or fully heal, painful leg ulcers in people with sickle cell disease (SCD), according to a post-hoc analysis of a Phase 3 trial.
These promising findings support the launch of future trials investigating the potential benefits of Oxbryta — already conditionally approved in the U.S. — at treating leg ulcers in these patients, researchers say.
Data from the post-hoc analysis was reported in “The Impact of Voxelotor Treatment on Leg Ulcers in Patients With Sickle Cell Disease,” a study published in the American Journal of Hematology.
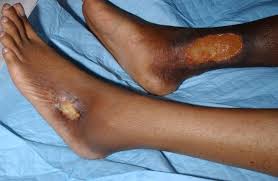
Leg ulcers are one of the most common and painful complications of SCD. In the U.S. alone, 14–18% of people with SCD are expected to develop leg ulcers at some point in their lives, and up to 5% are estimated to have active leg ulcers.
These wounds tend to appear in regions where blood flow is limited, and are more frequent in patients with severe anemia.
Despite being common and causing severe debilitation, there are no standard treatment protocols for leg ulcers in SCD patients.
Developed by Global Blood Therapeutics (GBT), Oxbryta is an oral medication conditionally approved in the U.S. to treat SCD in children and adults, ages 12 and older.
The medication is also under review for the same indication in Europe, while in the U.S., GBT is planning to request its use be expanded to include younger children, ages 4–11.
Oxbryta works by increasing hemoglobin’s affinity to oxygen, thereby preventing hemoglobin molecules from sticking to each other, deforming and destroying red blood cells — the root cause of SCD. Hemoglobin is a protein found inside red blood cells that is responsible for oxygen transport.
The therapy’s approval in the U.S., at a dose of 1,500 mg, was based on data from the HOPE Phase 3 trial (NCT03036813). That study compared the safety and efficacy of two daily doses of Oxbryta — 900 and 1,500 mg — to a placebo in 274 SCD patients, ages 12–65.
Data from the first six months of HOPE showed Oxbryta was able to increase the levels of hemoglobin in both children and adults with SCD and, at the higher dose, lower markers of red blood cell destruction.
More recently, GBT presented 72-week data from the study, which confirmed that a daily dose of 1,500 mg of Oxbryta led to sustained improvements in the levels of hemoglobin and disease markers. The therapy also was found to work better than a placebo at lowering the frequency of SCD pain crises.
By tackling the root cause of the disease, it is possible that Oxbryta also may be beneficial at alleviating leg ulcers in SCD patients.
To explore this possibility, GBT investigators and their colleagues in the U.S., Jamaica, and the Netherlands carried out a post-hoc analysis of HOPE that focused on the incidence of leg ulcers and clinical outcomes of these patients during the trial.
Over the study’s 72 weeks (nearly 1.5 years), a total of 22 patients (8%) had active leg ulcers. Among them, 13 already had active wounds at the study’s start, while the remaining nine developed leg ulcers during the trial.
Analyses showed that all five patients who had active leg ulcers and received the approved dose of Oxbryta saw their wounds either improve or fully heal over the course of the study. Eight of the nine (89%) patients who received the lower dose of Oxbryta also achieved the same outcome.
In contrast, only five of the eight (63%) patients in the placebo group with active leg ulcers saw their wounds partially or fully heal in the same period of time.
In more than 70% of patients treated with Oxbryta, leg ulcers resolved within a period of six months. Moreover, leg ulcer resolution was generally associated with increases in hemoglobin levels and reductions in the levels of disease markers.
“The results of this study suggest that voxelotor presents a potential clinical benefit for patients with SCD and leg ulcers and support future study in a prospective clinical trial,” the investigators wrote.




Recent Comments