GBT and Advocates Launch Disease Awareness Campaign Focused on Breaking Down Stigmas Associated with Sickle Cell Disease
Source: Global Blood Therapeutics, Inc.
SOUTH SAN FRANCISCO, Calif., July 10, 2019 (GLOBE NEWSWIRE) — Global Blood Therapeutics, Inc. (GBT) (NASDAQ: GBT), in partnership with sickle cell community-based organizations (CBOs), today announced the launch of Sickle Cell Speaks, a national campaign focused on breaking down stigmas associated with sickle cell disease (SCD).
Sickle Cell Speaks is designed to bring together a community of people, from patients to caregivers to friends and relatives, whose lives are affected by SCD. The campaign shares the individual experiences of those living with SCD through authentic stories from a diverse group of patients and caregivers. Sickle Cell Speaks aims to inspire hope by showcasing stories of strength and to dispel the misconceptions about SCD.
“The sickle cell patient experience is highly varied, and highlighting the personal stories of these individuals, as well as their family and friends, will help grow understanding and awareness of this condition,” said Beverly Francis-Gibson, president and chief executive officer of the Sickle Cell Disease Association of America. “We are proud to partner with GBT to help break down stigmas and make our voices heard.”
Additional partners of Sickle Cell Speaks include Sickle Cell Community Consortium, Sickle Cell 101, Bold Lips for Sickle Cell and Sickle Cell Warriors.
“We are partnering with several CBOs representing the sickle cell community to educate people about SCD and to help break down the barriers, particularly the misconceptions and social stigmas, that affect how those with SCD are viewed by society and which ultimately impact patients’ ability to access quality care,” said Ted W. Love, M.D., president and chief executive officer of GBT. “In addition to this patient-focused initiative, we recently launched a separate campaign aimed at engaging health care professionals and facilitating discussion around the root cause and molecular basis of the disease. Together, our patient and physician campaigns underscore GBT’s commitment to bringing together a broad range of stakeholders, with the goal of improving the lives of people living with sickle cell.”
GBT’s health care professional-focused disease awareness campaign, SCD Silent Damage, seeks to increase attention among physicians regarding hemoglobin polymerization, the molecular basis of SCD that initiates the sickling of red blood cells and the ensuing cascade of clinical complications that drive high levels of morbidity and mortality in patients.
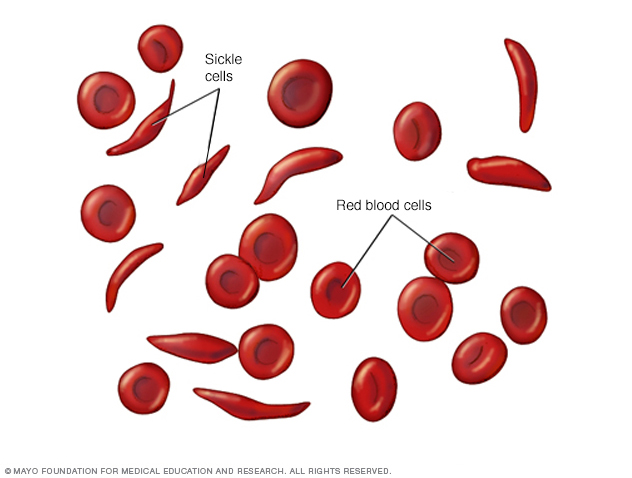
SCD is a rare, inherited disease that affects approximately 100,000 people in the United States. People of African descent make up 90 percent of this population, although it also affects people of Hispanic, South Asian, Southern European and Middle Eastern ancestry.1 Red blood cells are responsible for carrying oxygen throughout the body. In people with SCD, the normally round and flexible red blood cells become shaped like crescents because the hemoglobin within them polymerizes to form rods, which deform the cells. These rigid, crescent-shaped cells are not able to pass through small blood vessels and tend to break apart easily, leading to anemia, fatigue, episodes of pain and organ damage.2
About GBT
GBT is a clinical-stage biopharmaceutical company determined to discover, develop and deliver innovative treatments that provide hope to underserved patient communities. GBT is developing its lead product candidate, voxelotor, as an oral, once-daily therapy for sickle cell disease. To learn more, please visit www.gbt.com and follow the company on Twitter @GBT_news.
Forward-Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995, including statements about GBT’s development plans for voxelotor and the potential benefits of voxelotor for SCD patients and other statements containing the words “anticipate,” “planned,” “believe,” “forecast,” “estimated,” “expected,” and “intend,” among others. These forward-looking statements are based on GBT’s current expectations and actual results could differ materially. Statements we make in this press release may include statements that are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. We intend these forward-looking statements, including statements regarding our plan to submit an NDA for voxelotor under an accelerated regulatory approval pathway, the availability of, and sufficiency of our data to support, accelerated regulatory approval, the therapeutic potential and safety profile of voxelotor, including the potential to be a disease-modifying therapy for SCD, our plan to initiate a TCD confirmatory study, the potential for TCD flow velocity to serve as an acceptable primary endpoint in a confirmatory study, the potential for voxelotor to become a new standard of care for treating adolescents and adults with SCD, our ability to implement and complete our clinical development plans for voxelotor, the potential for an increase in hemoglobin of 1 g/dL or greater to reduce the risk of stroke and mortality in patients with SCD, our ability to generate and report data from our ongoing and potential future studies of voxelotor (including data from patients enrolled in our Phase 3 HOPE Study, and data from our ongoing Phase 2a HOPE-KIDS 1 Study), regulatory review and actions relating to voxelotor, our potential commercial launch of voxelotor, and the timing of these events, to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These forward-looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. We can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved, and furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control including, without limitation, the risks that our clinical and preclinical development activities may be delayed or terminated for a variety of reasons, that results of clinical trials may be subject to differing interpretations, that regulatory authorities may disagree with our clinical development plans or require additional studies or data to support further clinical investigation of our product candidates, that drug-related adverse events may be observed in clinical development, and that data and results may not meet regulatory requirements or otherwise be sufficient for further development, regulatory review or approval, along with those risks set forth in our Annual Report on Form 10-K for the fiscal year ended December 31, 2018, and in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2019, as well as discussions of potential risks, uncertainties and other important factors in our subsequent filings with the U.S. Securities and Exchange Commission. Except as required by law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
References
- Centers for Disease Control and Prevention. Sickle cell disease data & statistics. Page last reviewed: August 9, 2017. Available from: https://www.cdc.gov/ncbddd/sicklecell/data.html. Accessed on May 1, 2019.
- National Heart, Lung and Blood Institute. Sickle Cell Disease. Available from: https://www.nhlbi.nih.gov/health-topics/sickle-cell-disease. Accessed on May 1, 2019.
Contact Information:
Stephanie Yao (investors & media)
GBT
650-741-7730
investor@gbt.com



Recent Comments